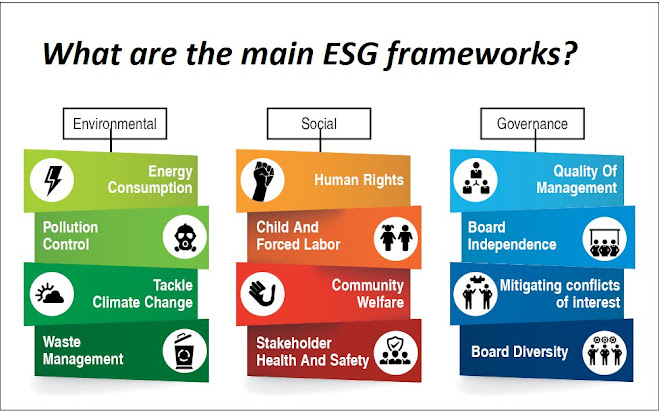
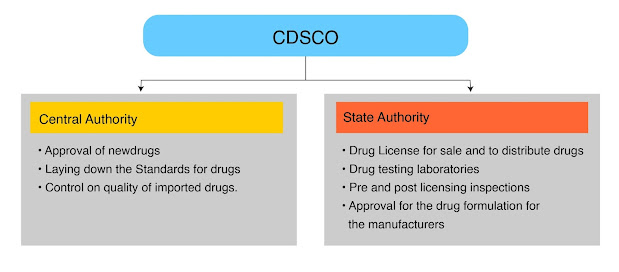
Why FSSAI State License is Essential for Your Food Business?

FSSAI State License is essential for any food business operating in India, as it is a mandatory requirement under the Food Safety and Standards Act, 2006. Here are some reasons why obtaining an FSSAI State License is crucial for your food business: Legal Compliance: FSSAI State License is a legal requirement for all food businesses in India. Failure to obtain this license can result in heavy penalties, fines, and even imprisonment. Ensures Food Safety: The FSSAI is responsible for ensuring the safety and quality of food products sold in India. By obtaining an FSSAI State License, your food business demonstrates its commitment to maintaining high food safety standards and complying with government regulations. Increases Consumer Trust: Consumers are becoming more aware of food safety issues and are looking for assurance that the food they eat is safe. An FSSAI State License assures consumers that your food business has met the government's strict food safety standards, which can in