What is the role of CDSCO?
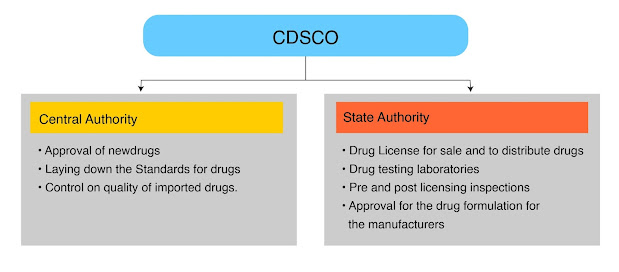
CDSCO (Central Drugs Standard Control Organization) is the national regulatory body in India responsible for regulating the import, manufacture, distribution, and sale of drugs, cosmetics, medical devices CDSCO, and diagnostics. It is the apex regulatory body under the Ministry of Health and Family Welfare, Government of India.
The primary role of CDSCO is to ensure that all drugs, cosmetics, medical devices, and diagnostics available in India are safe, effective, and of high quality. Some of the key functions of CDSCO include:
- Registration of drugs and medical devices: CDSCO is responsible for granting licenses to manufacturers and importers to ensure that they comply with the relevant quality standards and regulatory requirements.
- Inspection and surveillance: CDSCO conducts regular inspections of manufacturing facilities and quality control laboratories to ensure that drugs and medical devices are being manufactured and tested in accordance with good manufacturing practices.
- Quality control: CDSCO conducts testing of samples of drugs and medical devices to ensure that they comply with the relevant quality standards.
- Adverse event reporting: CDSCO monitors and investigates adverse events related to drugs and medical devices and takes appropriate action to protect public health.
- Regulatory oversight: CDSCO also provides guidance to industry on regulatory compliance and takes enforcement action when necessary to ensure compliance with relevant laws and regulations.
Who provides drug licenses in India?
Who can apply for CDSCO?
- In India, the Central Drugs Standard Control Organization (CDSCO) is responsible for regulating the import, manufacture, distribution and sale of drugs, cosmetics, medical devices, and diagnostics. The following entities can apply to the CDSCO for various licenses and approvals:
- Manufacturers of drugs and CDSCO medical devices: Manufacturers of drugs and medical devices can apply to the CDSCO for a manufacturing license, import license, or registration certificate for their products.
- Importers of drugs and medical devices: Importers of drugs and medical devices can apply to the CDSCO for an import license or registration certificate for their products.
- Distributors and wholesalers: Distributors and wholesalers of drugs and medical devices can apply to the CDSCO for a wholesale license.
- Clinical research organizations: Clinical research organizations (CROs) can apply to the CDSCO for approval to conduct clinical trials for drugs and medical devices.
- Hospitals and clinics: Hospitals and clinics can apply to the CDSCO for approval to manufacture drugs or medical devices for in-house use.
- Testing laboratories: Testing laboratories can apply to the CDSCO for accreditation to test drugs and medical devices.
How do I get a CDSCO certificate?
- Determine the type of certificate you need: CDSCO issues various certificates for drugs, medical devices, cosmetics, and diagnostics. Determine the specific type of certificate you need based on the nature of your product and its intended use.
- Ensure compliance with regulatory requirements: Before applying for a CDSCO certificate, ensure that your product complies with the regulatory requirements set forth by the CDSCO under the Drugs and Cosmetics Act, 1940 and the Drugs and Cosmetics Rules, 1945. This may include obtaining necessary licenses and approvals, conducting clinical trials, and complying with quality and safety standards.
- Submit an application: Once you have ensured compliance with regulatory requirements, you can submit an application for a CDSCO registration certificate to the relevant CDSCO office. The application form, along with supporting documents such as manufacturing licenses, clinical trial data, and quality control certificates, must be submitted as per the guidelines issued by the CDSCO.
- Pay the required fees: CDSCO charges fees for various services, including issuance of certificates. Ensure that you pay the required fees as per the fee schedule prescribed by the CDSCO.
- Await approval: Once your application is submitted, it will be reviewed by the CDSCO. If your application meets all the requirements, you will be issued a CDSCO certificate. The time taken to process the application can vary depending on the type of certificate and the volume of applications received by the CDSCO.
Why is CDSCO registration required?
- Protecting public health: CDSCO registration ensures that drugs, medical devices, cosmetics, and diagnostics available in India are safe and effective for human use, and do not pose any undue risks to public health.
- Regulatory compliance: CDSCO registration ensures that manufacturers, importers, and distributors of drugs, medical devices, cosmetics, and diagnostics comply with the relevant regulatory requirements set forth by the Indian government. This includes compliance with quality standards, labeling requirements, and packaging requirements.
- Improving product quality: CDSCO registration requires manufacturers to follow good manufacturing practices (GMP) to ensure that products are manufactured in a consistent and controlled manner, and are of high quality.
- Preventing fraudulent or substandard products: CDSCO registration helps to prevent the sale and distribution of fraudulent or substandard drugs, medical devices, cosmetics, and diagnostics in the Indian market.
- Promoting transparency and accountability: CDSCO registration requires manufacturers to disclose information about the ingredients, composition, and manufacturing process of their products, thereby promoting transparency and accountability in the pharmaceutical and medical device industries.


Comments
Post a Comment